Pituitary Pars Intermedia Dysfunction, or PPID, is an endocrine disease that can affect any horse, pony or donkey, regardless of breed, sex or management. It’s a progressive, degenerative disease, so incidence increases with age and it generally has a slow insidious onset, making it difficult to detect and diagnose in the early stage. The excess hormones produced affect various body systems, and individual horses can show different symptoms, some of which can be serious, like laminitis.
PPID used to be called Equine Cushing’s Disease, as there were thought to be similarities with Cushing’s Disease in humans and dogs, which involves a tumour that causes high levels of ACTH to be produced in the pars distalis of the pituitary gland, leading to high levels of cortisol, but no neurodegeneration. However, PPID in horses is different and is caused by neurodegeneration, affects the pars intermedia of the pituitary gland, and causes high levels of several hormones, but cortisol levels are often normal.
PPID used to be called Equine Cushing’s Disease, as there were thought to be similarities with Cushing’s Disease in humans and dogs, which involves a tumour that causes high levels of ACTH to be produced in the pars distalis of the pituitary gland, leading to high levels of cortisol, but no neurodegeneration. However, PPID in horses is different and is caused by neurodegeneration, affects the pars intermedia of the pituitary gland, and causes high levels of several hormones, but cortisol levels are often normal.
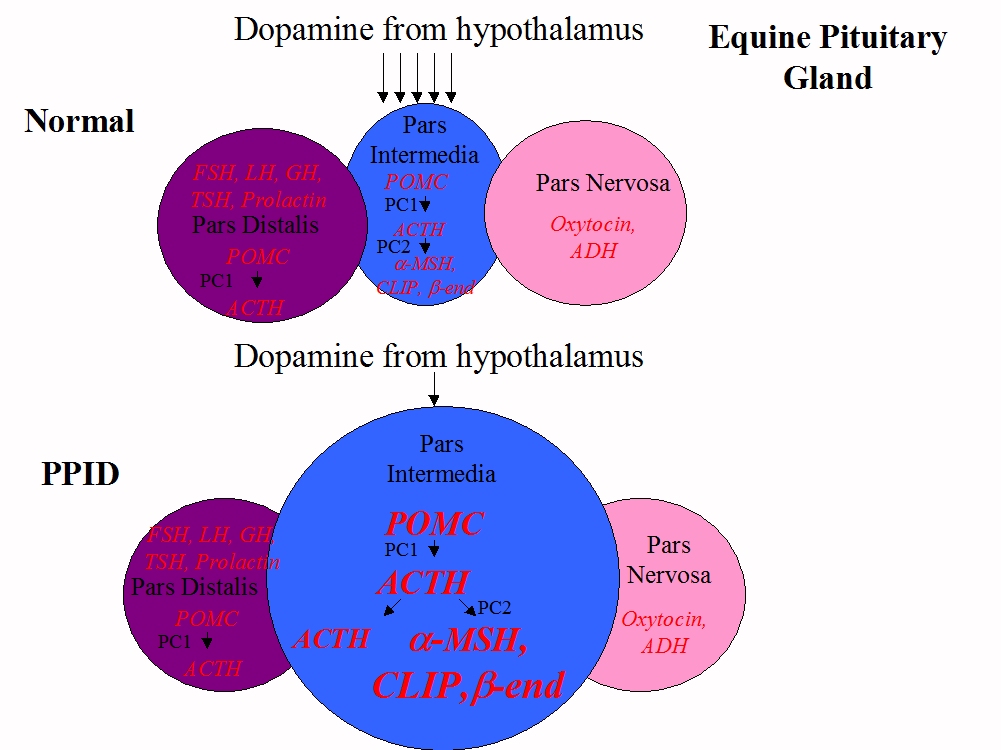
The pituitary gland lies below the hypothalamus at the bottom of the horse’s brain. It consists of three major hormone-releasing lobes: the pars distalis, the pars nervosa and the pars intermedia.
All horses, whether they have PPID or not, react to stress by releasing ACTH from the pars distalis into the blood. This tells the adrenal glands to release cortisol, which plays a role in the “fight or flight” response. The high levels of cortisol in the blood “feed back” on the pars distalis, reducing ACTH production.
In a normal horse, the pars intermedia plays a role in seasonal changes, metabolism and inflammation, producing a peptide called POMC, which is initially changed to ACTH, then around 98% of that ACTH is changed to alpha-MSH, beta-endorphin and CLIP before being released into the blood. This hormone production is slowed or stopped when neurons originating in the hypothalamus release dopamine (a neurotransmitter or chemical messenger) onto receptors on the pars intermedia. The pars intermedia is stimulated by the hormone TRH – this can be manipulated for diagnostic testing.
All horses, whether they have PPID or not, react to stress by releasing ACTH from the pars distalis into the blood. This tells the adrenal glands to release cortisol, which plays a role in the “fight or flight” response. The high levels of cortisol in the blood “feed back” on the pars distalis, reducing ACTH production.
In a normal horse, the pars intermedia plays a role in seasonal changes, metabolism and inflammation, producing a peptide called POMC, which is initially changed to ACTH, then around 98% of that ACTH is changed to alpha-MSH, beta-endorphin and CLIP before being released into the blood. This hormone production is slowed or stopped when neurons originating in the hypothalamus release dopamine (a neurotransmitter or chemical messenger) onto receptors on the pars intermedia. The pars intermedia is stimulated by the hormone TRH – this can be manipulated for diagnostic testing.
With PPID, the dopamine-producing neurons in the hypothalamus slowly degenerate, and with less dopamine to inhibit hormone production, the pars intermedia releases massively increased amounts of alpha-MSH, beta-endorphin, CLIP and also ACTH, which are thought to cause the clinical signs of PPID. The excess ACTH produced in horses with PPID may be less biologically active than normal, which may explain why horses with PPID often have normal cortisol levels, even when blood tests report high ACTH levels.
As in any factory, increased production leads to expansion - the pars intermedia cells increase in number (hyperplasia) and size (hypertrophy), often leading to adenoma (benign tumour) formation, and causing the pars intermedia to increase in size (a normal pituitary gland weighs around 2 g, in a horse with advanced PPID it can weigh more than 10 g), which may cause compression of the other lobes of the pituitary gland.
As in any factory, increased production leads to expansion - the pars intermedia cells increase in number (hyperplasia) and size (hypertrophy), often leading to adenoma (benign tumour) formation, and causing the pars intermedia to increase in size (a normal pituitary gland weighs around 2 g, in a horse with advanced PPID it can weigh more than 10 g), which may cause compression of the other lobes of the pituitary gland.
What causes PPID?
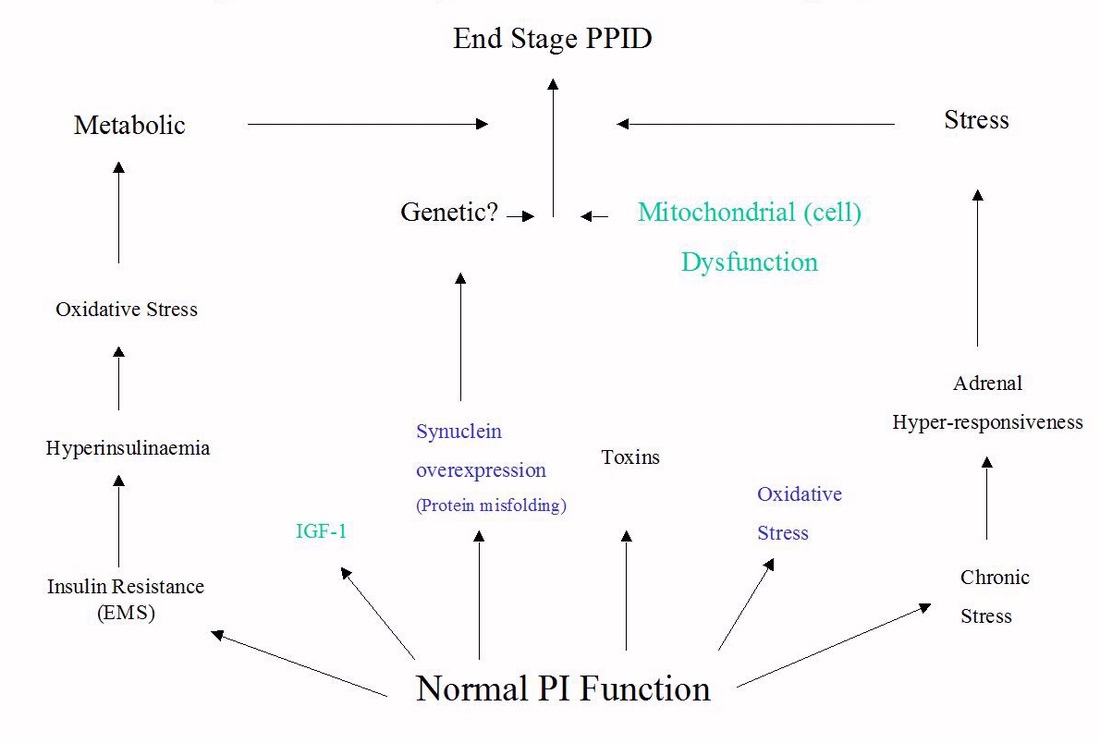
The cause of PPID is still unknown, but research suggests that localised oxidative stress may contribute to dopaminergic neuron damage and cell death. Dr Dianne McFarlane found decreased activity of the antioxidant manganese superoxide dismutase in the pars intermedia in older horses, which could contribute to the risk of PPID developing with age, and she also found evidence of protein misfolding in the pars intermedia of horses with PPID, similar to that found in Parkinson’s disease.
In her presentation on the Pathophysiology of PPID at the 2011 Equine Endocrinology Summit, Dr McFarlane suggested that there may be several syndomes that lead to PPID, such as metabolic disorders like EMS, toxins in the environment, stress or genetic predisposition. This might explain why individual horses with PPID can have differing symptoms and hormone levels.
The cause of PPID is still unknown, but research suggests that localised oxidative stress may contribute to dopaminergic neuron damage and cell death. Dr Dianne McFarlane found decreased activity of the antioxidant manganese superoxide dismutase in the pars intermedia in older horses, which could contribute to the risk of PPID developing with age, and she also found evidence of protein misfolding in the pars intermedia of horses with PPID, similar to that found in Parkinson’s disease.
In her presentation on the Pathophysiology of PPID at the 2011 Equine Endocrinology Summit, Dr McFarlane suggested that there may be several syndomes that lead to PPID, such as metabolic disorders like EMS, toxins in the environment, stress or genetic predisposition. This might explain why individual horses with PPID can have differing symptoms and hormone levels.
Diagnosis of PPID
Diagnosis of PPID is based on clinical signs and history, backed up by above normal ACTH blood test results. Although incidence increases with age, horses as young as 6 or 7 have been diagnosed with PPID.
Diagnosis of PPID is based on clinical signs and history, backed up by above normal ACTH blood test results. Although incidence increases with age, horses as young as 6 or 7 have been diagnosed with PPID.
Clinical signs
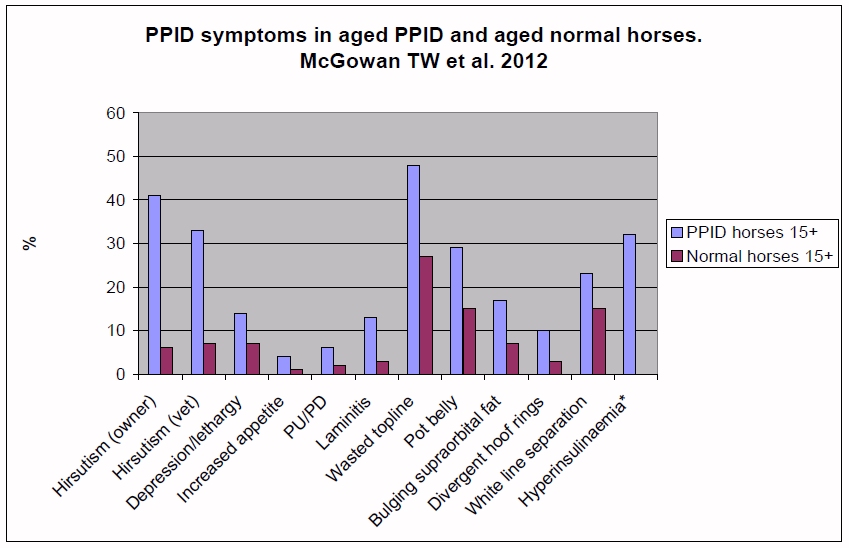
The clinical signs seen vary between horses and with the stage of the disease, and are often mistaken for normal ageing. Experts often group clinical signs into early or advanced stage, but there is likely to be overlap. Symptoms are often worse during the autumn “seasonal rise”.
Early signs may include:
The clinical signs seen vary between horses and with the stage of the disease, and are often mistaken for normal ageing. Experts often group clinical signs into early or advanced stage, but there is likely to be overlap. Symptoms are often worse during the autumn “seasonal rise”.
Early signs may include:
- Long hair on legs, neck and face.
- Delayed or patchy shedding of haircoat.
- Muscle loss along topline.
- Lethargy/depression/docility.
- Decreased athletic performance.
- Laminitis (usually with abnormal fat deposits and insulin dysregulation).
|
As the condition becomes more advanced, additional signs may include:
|
- Abnormal haircoat – from a thicker than normal summer coat, long coarse hairs in the coat or patches of long hair to a long shaggy coat (hypertrichosis) that doesn’t shed at all.
- Hyperglycaemia (above normal blood glucose concentrations).
- Neurological symptoms such as ataxia, seizures and blindness.
It has been considered that hypertrichosis – the long coat that doesn’t shed – is diagnostic of PPID, but whilst it is highly suggestive, there can be other causes, such as severe malnutrition.
Scooby on arrival at Remus Horse Sanctuary showing hypertrichosis and muscle loss, and after being clipped and starting treatment for PPID.
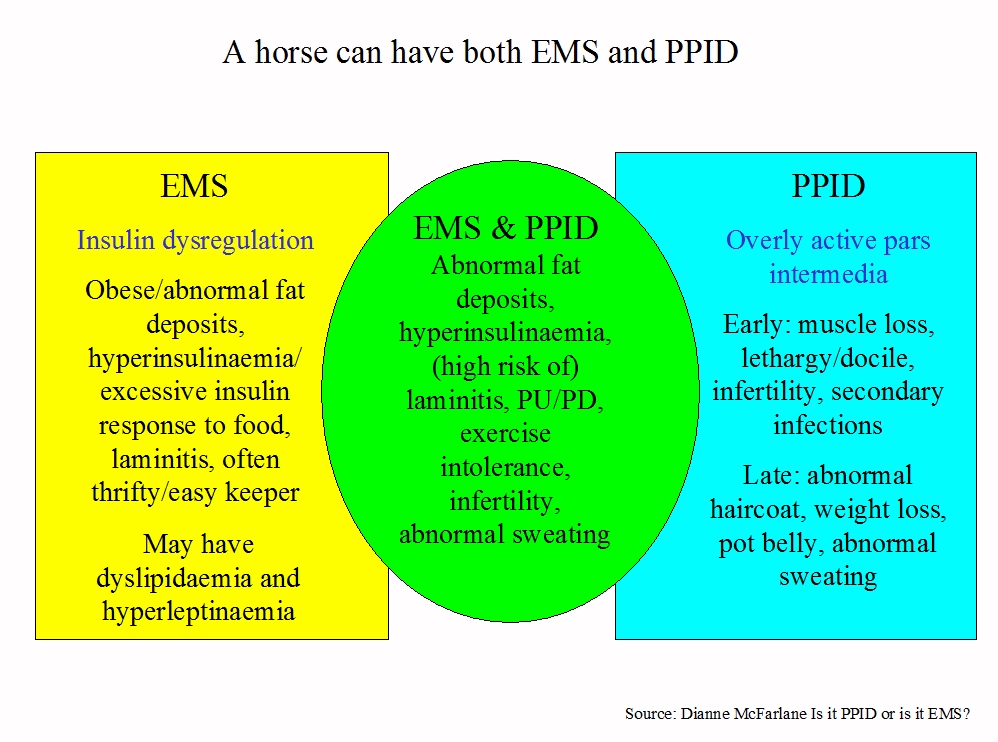
It is currently thought that some but not all horses with PPID will also be at risk of laminitis – they have PPID and Equine Metabolic Syndrome (EMS), and are likely to have insulin dysregulation and abnormal fat deposits, e.g. cresty neck, filled supra-orbital hollows and fat around the tailhead, in addition to other symptoms of PPID. To identify these horses and assess laminitis risk, insulin dysregulation should be measured in all horses suspected of having PPID. Horses with PPID that have never had laminitis and that have normal insulin may not be at greater risk of developing laminitis than any other horse.
Although it isn’t yet known whether having EMS causes PPID, it seems that horses with EMS may be at greater risk of developing PPID as they get older, and therefore horses with EMS should be monitored and tested for PPID, and their diet, weight and exercise addressed with the aim of reversing EMS. Vets are also noticing that there appears to be a transitional period as horses with EMS develop PPID during which their insulin concentrations and therefore risk of laminitis increase.
Blood tests
As PPID is a progressive disease, blood tests are often negative in the early stages, but false positives are also seen when ACTH is released as part of the normal stress response. Hormone levels are affected by seasons and geographical location, are released in pulses so concentrations can change within minutes, and vary considerably between affected horses.
As PPID is a progressive disease, blood tests are often negative in the early stages, but false positives are also seen when ACTH is released as part of the normal stress response. Hormone levels are affected by seasons and geographical location, are released in pulses so concentrations can change within minutes, and vary considerably between affected horses.
|
The Equine Endocrinology Group (EEG) has published recommendations for the diagnosis of PPID (http://sites.tufts.edu/equineendogroup/) and suggests initially testing:
Resting ACTH concentration, which requires a single blood draw and can be done at any time of the day or year, as long as seasonally adjusted reference ranges are used (see Liphook Equine Hospital's weekly cutoff values for ACTH), and the horse is not stressed before or during the blood collection. Note that ACTH can be measured using different assays, with different reference ranges, therefore results from laboratories may not be comparable. Horses in the UK that have not been diagnosed with PPID may be eligible for a free ACTH test – see http://www.talkaboutlaminitis.co.uk/. |
When resting ACTH results are borderline or negative despite a suspicion of PPID, the TRH stimulation of ACTH may be recommended. A resting ACTH blood sample is collected, then 1 mg of TRH is injected and a further ACTH blood sample collected 10 minutes later. Horses with PPID appear to produce more ACTH in response to an injection of TRH than normal horses. Currently this test cannot be used in the autumn, and more research is required to define normal reference ranges.
All horses suspected of having PPID should be tested for insulin dysregulation using either a resting insulin test, or if no clinical signs of insulin dysregulation are seen and/or if resting insulin test results are normal, an oral sugar test, to assess laminitis risk.
The dexamethasone suppression test, once considered the gold standard for PPID testing, is no longer recommended as it may cause laminitis, requires two vet visits, cannot be used in the autumn, and may only detect advanced cases. Tests measuring cortisol are not diagnostic for PPID.
All horses suspected of having PPID should be tested for insulin dysregulation using either a resting insulin test, or if no clinical signs of insulin dysregulation are seen and/or if resting insulin test results are normal, an oral sugar test, to assess laminitis risk.
The dexamethasone suppression test, once considered the gold standard for PPID testing, is no longer recommended as it may cause laminitis, requires two vet visits, cannot be used in the autumn, and may only detect advanced cases. Tests measuring cortisol are not diagnostic for PPID.
All horses have a “seasonal rise” in pars intermedia hormone production in the autumn, probably to help them prepare for the winter, which starts as days begin to get shorter and is considered significant from August to October. The increase is greater in horses with PPID, making this the best time to test ACTH, as long as seasonally adjusted reference ranges are used.
A diagnosis of PPID should only be made if there are clinical signs of PPID. There is no way of knowing whether the ACTH in a blood sample is from the pars intermedia and suggests PPID if abnormally high, or has been produced by the pars distalis as part of a normal stress response. “Stress” can be due to pain (e.g. laminitis), illness, excitement, exercise, travelling, use of a twitch, veterinary procedures e.g. dental work or “white coat phobia”. Some medicines may increase ACTH, e.g. clenbuterol (Ventipulmin) and some sedation durgs, and current advice (from Liphook Equine Hospital) is that ACTH should not be tested following sedation. Note also that freezing the blood sample before it has been separated by centrifuge can lead to falsely high ACTH results.
In the future, testing of other PPID hormones may be commercially available, which may make blood testing more accurate.
A diagnosis of PPID should only be made if there are clinical signs of PPID. There is no way of knowing whether the ACTH in a blood sample is from the pars intermedia and suggests PPID if abnormally high, or has been produced by the pars distalis as part of a normal stress response. “Stress” can be due to pain (e.g. laminitis), illness, excitement, exercise, travelling, use of a twitch, veterinary procedures e.g. dental work or “white coat phobia”. Some medicines may increase ACTH, e.g. clenbuterol (Ventipulmin) and some sedation durgs, and current advice (from Liphook Equine Hospital) is that ACTH should not be tested following sedation. Note also that freezing the blood sample before it has been separated by centrifuge can lead to falsely high ACTH results.
In the future, testing of other PPID hormones may be commercially available, which may make blood testing more accurate.
|
Treatment
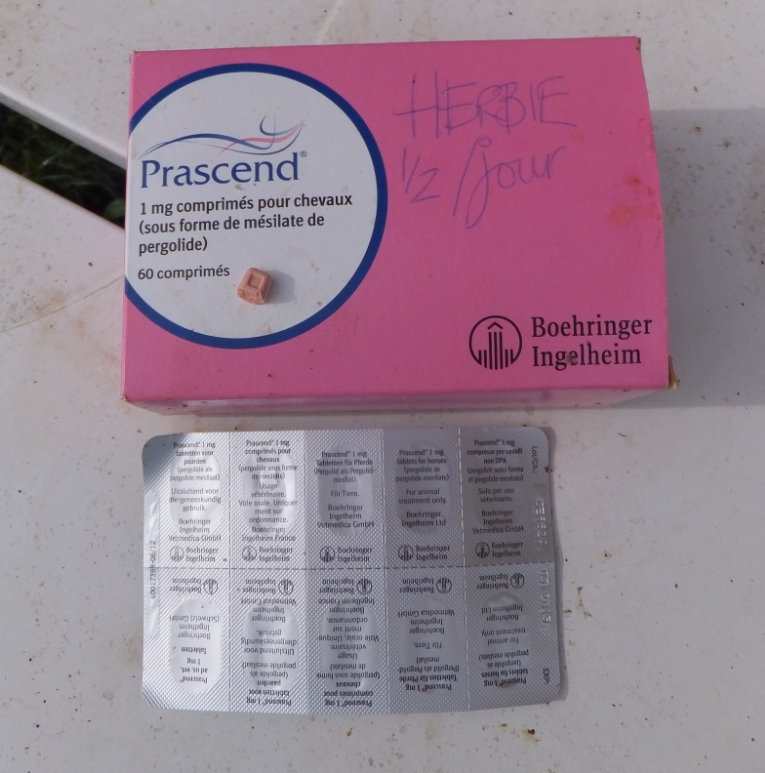
PPID currently cannot be cured, but can be treated with daily oral administration of a dopamine agonist such as pergolide (licensed for horses as Prascend in many countries), which replaces the missing dopamine, signalling the pars intermedia to reduce hormone production, and thereby reducing the clinical signs of PPID. It isn’t currently known whether treatment with pergolide will prevent or slow the hyperplasia and hypertrophy associated with the excess hormone production of the pars intermedia, but in theory this seems possible. In addition, research by Gille et al. (2002) found that pergolide protects dopamine-producing neurons under conditions of elevated oxidative stress, and Dr McFarlane has theorised that any antioxidant and neuroprotective properties of pergolide could be beneficial in slowing the progression of PPID, suggesting that early treatment with pergolide may be advisable. |
The initial dose of Prascend recommended is 0.002 mg/kg bodyweight once a day, so 1 mg for a 500 kg horse, but the dose may depend on the stage of the PPID, the season and other factors, and it should be titrated to the lowest effective dose for each individual horse based on response to therapy, whether that is improvement in clinical signs and blood results or signs of intolerance. Many horses require an increased dose during the seasonal rise, with a subsequent reduction in dose around Christmas.
Some horses go off their food or become depressed when starting treatment with pergolide, but when introduced gradually, ideally starting with 0.25 mg and slowly increasing to the recommended dose, side effects are minimised. Giving pergolide at different times to bucket feeds has helped some horses overcome inappetence.
Response to treatment is individual, but the EEG suggests that within 30 days of starting treatment an improvement in lethargy and depression, PU/PD and blood glucose concentrations should be seen, with improvements in haircoat abnormalities, improved topline and reduced incidences of laminitis and infections within a year. In The Laminitis Site’s experience, improvements are often noticed sooner than this, particularly when the PPID has not yet reached the advanced stage.
Some horses go off their food or become depressed when starting treatment with pergolide, but when introduced gradually, ideally starting with 0.25 mg and slowly increasing to the recommended dose, side effects are minimised. Giving pergolide at different times to bucket feeds has helped some horses overcome inappetence.
Response to treatment is individual, but the EEG suggests that within 30 days of starting treatment an improvement in lethargy and depression, PU/PD and blood glucose concentrations should be seen, with improvements in haircoat abnormalities, improved topline and reduced incidences of laminitis and infections within a year. In The Laminitis Site’s experience, improvements are often noticed sooner than this, particularly when the PPID has not yet reached the advanced stage.
|
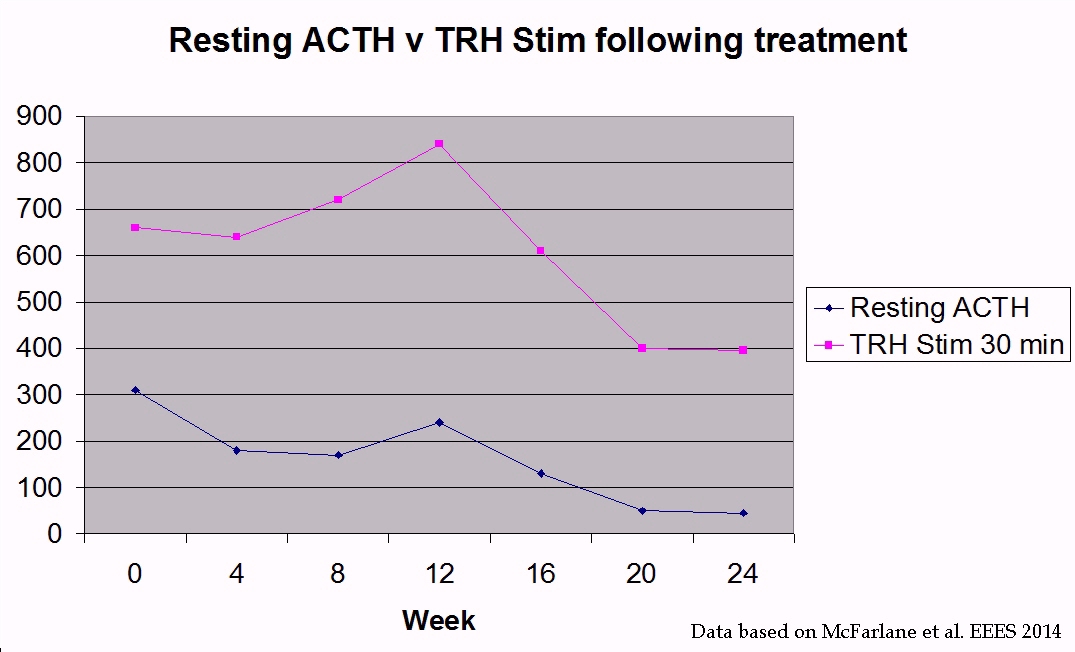
Blood should be tested 30 - 60 days after starting pergolide to assess response to treatment. PPID horses treated with pergolide and retested every 4 weeks showed significant reductions in resting ACTH levels, but TRH stimulation of ACTH results rose initially, suggesting resting ACTH may be more suitable for monitoring response to treatment.
It is suggested that horses with PPID have blood tests every 6 months, with one test during the seasonal rise (ideally August), to monitor the progression of the disease and their response to treatment. |
Currently there is no research that supports the use of herbal, homeopathic or other non-medical treatments for PPID. Some owners report improvements in depression and coat shedding with Vitex agnus castus, but research by Jill Beech et al. (2002) found no improvement in hormone levels and that clinical signs sometimes worsened when PPID horses were treated with Vitex agnus castus.
Management is also very important and should be optimized. Excess hair should be clipped and rugs used to help regulate the horse’s body temperature. The diet should contain above minimum levels of quality protein, minerals, vitamins and essential fatty acids, with energy levels appropriate for the horse’s lifestyle, and sugar and starch amounts kept low if the horse has insulin dysregulation. In theory feeds high in antioxidants could help to slow the neurodegenerative process that causes PPID, but there is currently no evidence for this. Attention should be paid to dental care, foot care, worming and vaccinations, and infections should be treated without delay.
Conclusion
Whilst there is still a lot to learn about PPID, recent advances in our knowledge have enabled horses to be diagnosed and start treatment much earlier in the disease process, with the result that many horses with PPID are living quality lives well into old age.
Whilst there is still a lot to learn about PPID, recent advances in our knowledge have enabled horses to be diagnosed and start treatment much earlier in the disease process, with the result that many horses with PPID are living quality lives well into old age.
References:
Is it PPID or EMS? Diagnosing Equine Endocrine Disease – a presentation by Dr Dianne McFarlane which can be viewed at www.thehorse.com
Equine Endocrinology Group
Recommendations for the Diagnosis and Treatment of Pituitary Pars Intermedia Dysfunction (PPID) 2014
http://sites.tufts.edu/equineendogroup/
AAEP proceedings Vol 48 2002
Comparison of Vitex agnus castus Extract and Pergolide in Treatment of Equine Cushing’s Syndrome
Beech J, Donaldson MT, Lindborg S
J Neural Transm. 2002 May;109(5-6):633-43
Pergolide protects dopaminergic neurons in primary culture under stress conditions
Gille G, Rausch WD, Hung ST, Moldzio R, Janetzky B, Hundemer HP, Kolter T, Reichmann H
2nd European Equine Endocrinology Symposium 14-16 May 2014
The use of ACTH response to TRH stimulation for monitoring horses with PPID and those receiving long-term pergolide
McFarlane D, Banse HE, J Fredrick, Yang F
Equine Endocrinology Summit 2011
Pathophysiology of Pituitary Pars Intermedia Dysfunction in 2011
Dianne McFarlane
Equine Veterinary Journal Vol45, Issue 1, pages 74–79, January 2013 (published online May 2012)
Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses
McGowan TW, Pinchbeck GP, McGowan CM
CAB Reviews 2015 10. No. 002
Equine Endocrinology
Menzies-Gow NJ
Is it PPID or EMS? Diagnosing Equine Endocrine Disease – a presentation by Dr Dianne McFarlane which can be viewed at www.thehorse.com
Equine Endocrinology Group
Recommendations for the Diagnosis and Treatment of Pituitary Pars Intermedia Dysfunction (PPID) 2014
http://sites.tufts.edu/equineendogroup/
AAEP proceedings Vol 48 2002
Comparison of Vitex agnus castus Extract and Pergolide in Treatment of Equine Cushing’s Syndrome
Beech J, Donaldson MT, Lindborg S
J Neural Transm. 2002 May;109(5-6):633-43
Pergolide protects dopaminergic neurons in primary culture under stress conditions
Gille G, Rausch WD, Hung ST, Moldzio R, Janetzky B, Hundemer HP, Kolter T, Reichmann H
2nd European Equine Endocrinology Symposium 14-16 May 2014
The use of ACTH response to TRH stimulation for monitoring horses with PPID and those receiving long-term pergolide
McFarlane D, Banse HE, J Fredrick, Yang F
Equine Endocrinology Summit 2011
Pathophysiology of Pituitary Pars Intermedia Dysfunction in 2011
Dianne McFarlane
Equine Veterinary Journal Vol45, Issue 1, pages 74–79, January 2013 (published online May 2012)
Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses
McGowan TW, Pinchbeck GP, McGowan CM
CAB Reviews 2015 10. No. 002
Equine Endocrinology
Menzies-Gow NJ
Abbreviations:
Alpha-MSH – alpha-melanocyte-stimulating hormone
ACTH – adrenocorticotropic hormone
CLIP – corticotrophin-like intermediate peptide
EEG – Equine Endocrinology Group
EMS – Equine Metabolic Syndrome
PPID – Pituitary Pars Intermedia Dysfunction
POMC – pro-opiomelanocortin
TRH – thyrotropin-releasing hormone
Alpha-MSH – alpha-melanocyte-stimulating hormone
ACTH – adrenocorticotropic hormone
CLIP – corticotrophin-like intermediate peptide
EEG – Equine Endocrinology Group
EMS – Equine Metabolic Syndrome
PPID – Pituitary Pars Intermedia Dysfunction
POMC – pro-opiomelanocortin
TRH – thyrotropin-releasing hormone
This article was originally published in The Arabian Magazine July 2015 and has been updated since publication.





 RSS Feed
RSS Feed