Sometimes ACTH produced normally by the pars distalis (PD) as part of the hypothalamic-pituitary-adrenal (HPA) axis appears to be confused with ACTH produced by the pars intermedia (PI) due to lack of dopaminergic inhibition in horses with PPID.
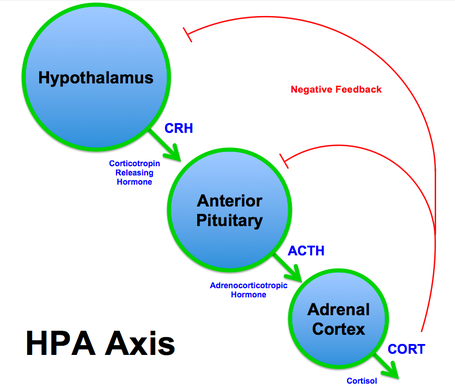
(Above image by Brian M Sweis - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=23363130)
All horses, whether they have PPID or not, react to a stress stimulus as follows:
CRH and AVP (vasopressin) are released by paraventricular neurons in the hypothalamus.
CRH and AVP stimulate corticotropes in the pars distalis (or anterior pituitary) of the pituitary gland to secrete ACTH.
ACTH travels in the blood to the adrenal glands, on top of the kidneys, where it stimulates the adrenal glands to release cortisol.
Cortisol is carried in the blood back to the hypothalamus and the pars distalis of the pituitary gland, which recognize the high levels of cortisol, and decrease or turn off CRH, AVP and ACTH release - this is called negative feedback, it prevents excessive hormone production.
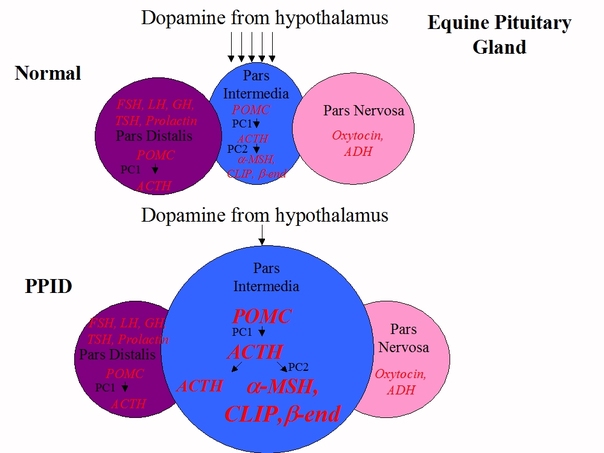
In a normal horse, the pars intermedia produces a peptide called POMC, which is changed to ACTH by an enzyme, PC1, then nearly all the ACTH is changed into alpha-MSH, beta-endorphin, CLIP and other hormones by another enzyme, PC2. Hormone production in the PI is controlled by dopamine, released by hypothalamic periventricular dopaminergic neurons, interacting with D2 dopamine receptors on the PI.
CRH and AVP (vasopressin) are released by paraventricular neurons in the hypothalamus.
CRH and AVP stimulate corticotropes in the pars distalis (or anterior pituitary) of the pituitary gland to secrete ACTH.
ACTH travels in the blood to the adrenal glands, on top of the kidneys, where it stimulates the adrenal glands to release cortisol.
Cortisol is carried in the blood back to the hypothalamus and the pars distalis of the pituitary gland, which recognize the high levels of cortisol, and decrease or turn off CRH, AVP and ACTH release - this is called negative feedback, it prevents excessive hormone production.
In a normal horse, the pars intermedia produces a peptide called POMC, which is changed to ACTH by an enzyme, PC1, then nearly all the ACTH is changed into alpha-MSH, beta-endorphin, CLIP and other hormones by another enzyme, PC2. Hormone production in the PI is controlled by dopamine, released by hypothalamic periventricular dopaminergic neurons, interacting with D2 dopamine receptors on the PI.
In horses with PPID, dopamine producing neurons are lost, and with less dopamine to inhibit hormone production, the PI releases massively increased amounts of alpha-MSH, beta-endorphin and CLIP, and also ACTH.
In a normal horse, only around 2% of circulating ACTH is made in the pars intermedia, 98% comes from the pars distalis, and dopamine plays no part in controlling normal ACTH output from the PD.
The excess levels of alpha-MSH, beta-endorphin and CLIP are thought to contribute to causing the clinical signs of PPID. The plasma ACTH in PPID horses is thought to be less biologically active than plasma ACTH in normal horses, which may explain why horses with PPID rarely have above normal cortisol levels. The lack of dopamine and increase in POMC-derived hormones leads to an increase in the number (hyperplasia) and size (hypertrophy) of the cells in the PI, eventually leading to adenoma (tumour) formation - this has nothing to do with cortisol.
The precise cause of PPID is still unknown, but research suggests that localised oxidative stress may contribute to dopaminergic neuron damage and death. However in 2005 Dianne McFarlane found that "there was no evidence of systemic accumulation of oxidative stress markers or deficiencies in antioxidant capacity in horses with PPID, suggesting that these are unlikely to be major predisposing factors in the development of PPID" (Systemic and pituitary pars intermedia antioxidant capacity associated with pars intermedia oxidative stress and dysfunction in horses).
In her 2011 paper on Equine PPID, Dr McFarlane states that pituitary antioxidant capacity has not been shown to be impaired in horses with PPID, but that the impairment of the activity of pituitary manganese superoxide dismutase found in older horses may contribute to the risk of PPID developing with age.
She goes on to say that excellent nutrition is important for horses with PPID, and that in theory, feeds high in antioxidants could slow the neurodegenerative process associated with PPID, but that there is currently no evidence for this. Early treatment with pergolide to replace the missing dopamine and reduce excess hormone production and the clinical signs of PPID is advised. Interestingly, research by Gille et al. (2002) found that "pergolide protects dopaminergic neurons under conditions of elevated oxidative stress"; similarly research by Uberti et al. in the same year suggested that "pergolide ... may interfere with the early phases of the oxidative stress-induced neurotoxic process". Dr McFarlane theorises that any antioxidant and neuroprotective properties of pergolide could be beneficial in slowing the progression of PPID.
So in conclusion, optimal management and treatment with pergolide is recommended for horses with PPID, but it appears that further research is needed before we can say for sure exactly what might slow the progression of this common equine neurodegenerative disease.
In a normal horse, only around 2% of circulating ACTH is made in the pars intermedia, 98% comes from the pars distalis, and dopamine plays no part in controlling normal ACTH output from the PD.
The excess levels of alpha-MSH, beta-endorphin and CLIP are thought to contribute to causing the clinical signs of PPID. The plasma ACTH in PPID horses is thought to be less biologically active than plasma ACTH in normal horses, which may explain why horses with PPID rarely have above normal cortisol levels. The lack of dopamine and increase in POMC-derived hormones leads to an increase in the number (hyperplasia) and size (hypertrophy) of the cells in the PI, eventually leading to adenoma (tumour) formation - this has nothing to do with cortisol.
The precise cause of PPID is still unknown, but research suggests that localised oxidative stress may contribute to dopaminergic neuron damage and death. However in 2005 Dianne McFarlane found that "there was no evidence of systemic accumulation of oxidative stress markers or deficiencies in antioxidant capacity in horses with PPID, suggesting that these are unlikely to be major predisposing factors in the development of PPID" (Systemic and pituitary pars intermedia antioxidant capacity associated with pars intermedia oxidative stress and dysfunction in horses).
In her 2011 paper on Equine PPID, Dr McFarlane states that pituitary antioxidant capacity has not been shown to be impaired in horses with PPID, but that the impairment of the activity of pituitary manganese superoxide dismutase found in older horses may contribute to the risk of PPID developing with age.
She goes on to say that excellent nutrition is important for horses with PPID, and that in theory, feeds high in antioxidants could slow the neurodegenerative process associated with PPID, but that there is currently no evidence for this. Early treatment with pergolide to replace the missing dopamine and reduce excess hormone production and the clinical signs of PPID is advised. Interestingly, research by Gille et al. (2002) found that "pergolide protects dopaminergic neurons under conditions of elevated oxidative stress"; similarly research by Uberti et al. in the same year suggested that "pergolide ... may interfere with the early phases of the oxidative stress-induced neurotoxic process". Dr McFarlane theorises that any antioxidant and neuroprotective properties of pergolide could be beneficial in slowing the progression of PPID.
So in conclusion, optimal management and treatment with pergolide is recommended for horses with PPID, but it appears that further research is needed before we can say for sure exactly what might slow the progression of this common equine neurodegenerative disease.
For more information, join Friends of The Laminitis Site and access our Facebook discussion/support group Friends of The Laminitis Site 1.

 RSS Feed
RSS Feed