Is it PPID or is it EMS – Diagnosing Equine Endocrine Disease
Dr Dianne McFarlane – Oklahoma State University - www.thehorse.com - 31 January 2014
These notes are based on this presentation but are not intended to be an accurate representation – no responsibility is taken for their accuracy.
Dr Dianne McFarlane – Oklahoma State University - www.thehorse.com - 31 January 2014
These notes are based on this presentation but are not intended to be an accurate representation – no responsibility is taken for their accuracy.
There are two common equine endocrine diseases:
Equine Pituitary Pars Intermedia Dysfunction – PPID
Equine Metabolic Syndrome – EMS
PPID
Pathophysiology of PPID (i.e. what goes wrong)
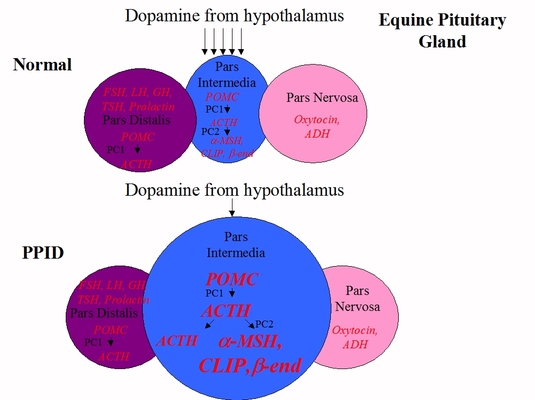
The equine pituitary gland is suspended from the hypothalamus (towards the bottom of the brain) by the infundibular stalk. It has 4 lobes:
Pars tuberalis – a thin band of tissue around the infundibular stalk;
Pars nervosa – secretes oxytocin and ADH (vasopressin);
Pars distalis (anterior lobe) - secretes many hormones including reproductive hormones: FSH, LH, GH, TSH, Prolactin, ACTH;
Pars intermedia.
The pars intermedia is made up of melanotrope endocrine cells. Hormone production is reduced when dopamine, released by periventricular dopaminergic neurons which originate in the hypothalamus, interacts with D2 dopamine receptors on the melanotropes of the pars intermedia.
Equine Pituitary Pars Intermedia Dysfunction – PPID
Equine Metabolic Syndrome – EMS
PPID
Pathophysiology of PPID (i.e. what goes wrong)
The equine pituitary gland is suspended from the hypothalamus (towards the bottom of the brain) by the infundibular stalk. It has 4 lobes:
Pars tuberalis – a thin band of tissue around the infundibular stalk;
Pars nervosa – secretes oxytocin and ADH (vasopressin);
Pars distalis (anterior lobe) - secretes many hormones including reproductive hormones: FSH, LH, GH, TSH, Prolactin, ACTH;
Pars intermedia.
The pars intermedia is made up of melanotrope endocrine cells. Hormone production is reduced when dopamine, released by periventricular dopaminergic neurons which originate in the hypothalamus, interacts with D2 dopamine receptors on the melanotropes of the pars intermedia.
The pars intermedia produces a peptide called POMC. Two enzymes, PC1 and PC2, cut the POMC into smaller hormones including alpha-MSH, CLIP and beta-endorphin.
In a normal horse, hardly any ACTH is made in the pars intermedia – it comes from the corticotrope cells of the pars distalis where POMC is cleaved into ACTH by PC1. PPID horses produce ACTH in both the pars distalis and the pars intermedia.
PPID is a dopaminergic neurodegenerative disease – the dopamine producing neurons are lost, and with less dopamine to inhibit hormone production, the pars intermedia releases massively increased amounts of alpha-MSH, beta-endorphin and CLIP, and also ACTH. This leads to hypertrophy (increase in cell size) and hyperplasia (increase in cell number), causing the pars intermedia and therefore the pituitary gland to increase in size – a normal pituitary gland weighs around 2 grams, in a horse with advanced PPID the pituitary gland can weigh more than 10 grams.
If the missing dopamine is replaced with a dopamine agonist, e.g. pergolide, hormone abnormalities and clinical signs of PPID are reduced.
Clinical signs of PPID
A collection of clinical signs can be seen in horses with PPID, some early and others later as the disease progresses.
Early:
Muscle loss
Lethargy/docile – horse becomes more mellow, perhaps nicer (due to increased beta-endorphin)
Infertility in breeding horses
Secondary infections e.g. sinusitis, abscesses
Late:
Abnormal haircoat – including regional abnormalities like long hair on the legs, long tufts on chin and belly which may shed, and hypertrichosis (failure to shed).
Profound weight loss – loss of all fat, not just muscle
Pot belly – due to loss of muscle tone
Abnormal sweating/thermoregulation - hyperhydrosis (increased sweating) or anhydrosis (lack of normal sweating in hot conditions).
In a normal horse, hardly any ACTH is made in the pars intermedia – it comes from the corticotrope cells of the pars distalis where POMC is cleaved into ACTH by PC1. PPID horses produce ACTH in both the pars distalis and the pars intermedia.
PPID is a dopaminergic neurodegenerative disease – the dopamine producing neurons are lost, and with less dopamine to inhibit hormone production, the pars intermedia releases massively increased amounts of alpha-MSH, beta-endorphin and CLIP, and also ACTH. This leads to hypertrophy (increase in cell size) and hyperplasia (increase in cell number), causing the pars intermedia and therefore the pituitary gland to increase in size – a normal pituitary gland weighs around 2 grams, in a horse with advanced PPID the pituitary gland can weigh more than 10 grams.
If the missing dopamine is replaced with a dopamine agonist, e.g. pergolide, hormone abnormalities and clinical signs of PPID are reduced.
Clinical signs of PPID
A collection of clinical signs can be seen in horses with PPID, some early and others later as the disease progresses.
Early:
Muscle loss
Lethargy/docile – horse becomes more mellow, perhaps nicer (due to increased beta-endorphin)
Infertility in breeding horses
Secondary infections e.g. sinusitis, abscesses
Late:
Abnormal haircoat – including regional abnormalities like long hair on the legs, long tufts on chin and belly which may shed, and hypertrichosis (failure to shed).
Profound weight loss – loss of all fat, not just muscle
Pot belly – due to loss of muscle tone
Abnormal sweating/thermoregulation - hyperhydrosis (increased sweating) or anhydrosis (lack of normal sweating in hot conditions).
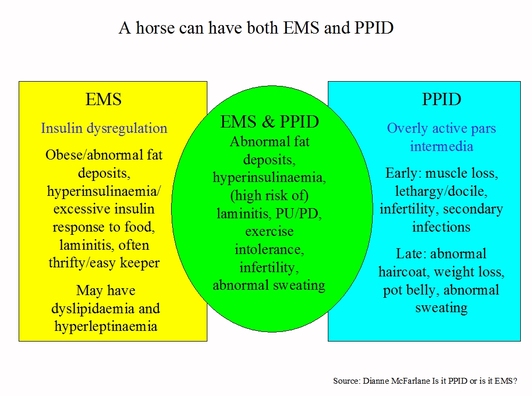
Some but not all horses with PPID will also have the potential for laminitis, hyperinsulinaemia, abnormal fat deposits, PU/PD (excessive drinking and urination), exercise intolerance, infertility, abnormal sweating – they have PPID and EMS. Some experts believe that not all horses with PPID are at risk from laminitis – only a subset of horses with PPID are at risk from laminitis (therefore insulin dysregulation should be measured in all horses with PPID to assess laminitis risk).
Diagnosis of PPID
Early stage PPID can be hard to diagnose – blood tests are often negative early in the condition, and clinical signs can overlap with normal aging. However, it is worth pursuing a positive test result to provide a useful guide for response to treatment.
A diagnosis of PPID should only be made if there are clinical signs of PPID, based on examination and a complete history – owners should regularly record weight, body condition score, dates of shedding/haircoat changes, how often feet need trimming, signs of laminitis and PPID such as hoof rings, to help the vet reach a diagnosis.
A long haircoat is highly suggestive of PPID in an older horse but it is not an absolute diagnosis – e.g. malnutrition can cause haircoat changes and false positive blood results. Concurrent disease can also affect clinical signs and blood results.
Diagnostic tests for PPID
Resting plasma ACTH concentration is now the most common test - the vet collects blood into a purple topped tube (EDTA). Equine ACTH is not particularly unstable and as long as it is kept cool it can be separated up to 12 hours later, and then kept chilled or frozen until it is tested.
There are 2 methods for measuring ACTH:
Chemiluminescent (CIA) - Immulite as used by Cornell and Liphook
Radioimmunoassay (RIA)
Reference ranges are different between CIA and RIA (and potentially between different RIA assays) – seasonally adjusted reference ranges specific to the testing lab must be used, and results may not be comparable between labs.
There is no way of telling whether the ACTH measured came from the pars intermedia or the pars distalis - ACTH may increase with other diseases or stress.
(TLS comment: more than one ACTH sample will increase diagnostic accuracy as ACTH concentrations can fluctuate significantly).
The dexamethasone suppression test (DST) is no longer recommended – there is a risk of causing/exacerbating laminitis, it requires 2 vet visits, it cannot be used in the autumn, and it may only detect advanced PPID cases.
Diagnosis of PPID
Early stage PPID can be hard to diagnose – blood tests are often negative early in the condition, and clinical signs can overlap with normal aging. However, it is worth pursuing a positive test result to provide a useful guide for response to treatment.
A diagnosis of PPID should only be made if there are clinical signs of PPID, based on examination and a complete history – owners should regularly record weight, body condition score, dates of shedding/haircoat changes, how often feet need trimming, signs of laminitis and PPID such as hoof rings, to help the vet reach a diagnosis.
A long haircoat is highly suggestive of PPID in an older horse but it is not an absolute diagnosis – e.g. malnutrition can cause haircoat changes and false positive blood results. Concurrent disease can also affect clinical signs and blood results.
Diagnostic tests for PPID
Resting plasma ACTH concentration is now the most common test - the vet collects blood into a purple topped tube (EDTA). Equine ACTH is not particularly unstable and as long as it is kept cool it can be separated up to 12 hours later, and then kept chilled or frozen until it is tested.
There are 2 methods for measuring ACTH:
Chemiluminescent (CIA) - Immulite as used by Cornell and Liphook
Radioimmunoassay (RIA)
Reference ranges are different between CIA and RIA (and potentially between different RIA assays) – seasonally adjusted reference ranges specific to the testing lab must be used, and results may not be comparable between labs.
There is no way of telling whether the ACTH measured came from the pars intermedia or the pars distalis - ACTH may increase with other diseases or stress.
(TLS comment: more than one ACTH sample will increase diagnostic accuracy as ACTH concentrations can fluctuate significantly).
The dexamethasone suppression test (DST) is no longer recommended – there is a risk of causing/exacerbating laminitis, it requires 2 vet visits, it cannot be used in the autumn, and it may only detect advanced PPID cases.
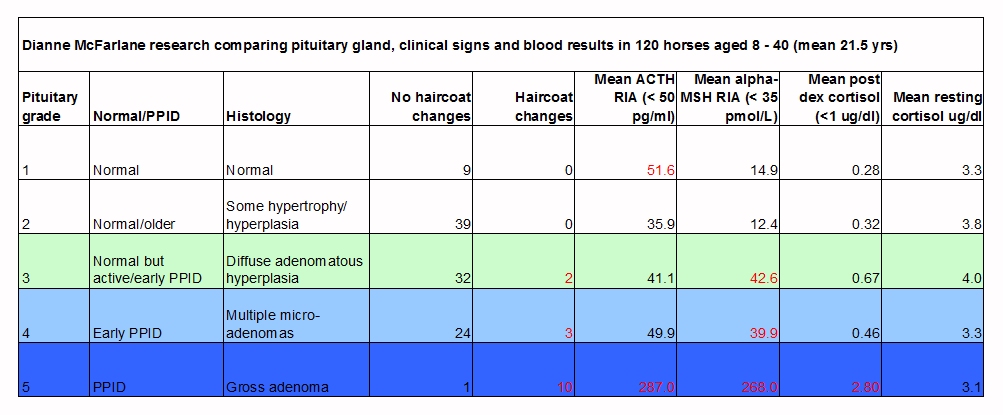
Dr McFarlane compared ACTH, alpha-MSH, dexamethasone suppression test (DST) cortisol and resting cortisol to pituitary gland cell changes and haircoat changes in 120 older horses, using the pituitary grading scale published by Miller:
Miller MA, Pardo ID, Jackson LP, Moore GE, Sojka JE
Correlation of Pituitary Histomorphometry with Adrenocorticotrophic Hormone Response to Domperidone Administration in the Diagnosis of Equine Pituitary Pars Intermedia Dysfunction
Vet Pathol 45:26–38 (2008)
Findings:
Sensitivity (true positives) Specificity (true negatives)
Alpha-MSH 63% 90%
ACTH 71% 81%
DST 65% 98%
All 3 tests identified advanced (grade 5) PPID.
All 3 tests were poor at identifying early PPID.
ACTH had a weaker correlation with pars intermedia enlargement than Alpha-MSH or DST.
Alpha-MSH had 2/48 false positives, ACTH had 13/48 false positives.
ACTH may be from the pars intermedia or the pars distalis, and may increase with other diseases or stress.
Resting cortisol is not predictive of PPID (this has been known for a long time).
A better test was needed.
Since around 2011 the TRH stimulation of ACTH has been used to diagnose PPID, and further research is being carried out on this test.
The vet collects a blood sample to measure resting ACTH, then injects 1 mg of the hormone TRH intravenously and collects blood to measure ACTH 10 and/or 30 mins after giving the TRH.
Currently suggested reference ranges are:
PPID if ACTH > 36 pg/ml (using Immulite CIA) at 0 or 30 minutes
PPID if ACTH > 110 pg/ml at 10 minutes
However these reference ranges are likely to change as more data is collected (normal horses have tested above these ranges), and reference ranges have not been established for the autumn seasonal rise.
Sensitivity (true positives) 88-95%, specificity (true negatives) 71-91% so the TRH stimulation of ACTH appears more diagnostic than resting ACTH.
TRH stimulates pars intermedia activity – however, increased activity does not necessarily mean dysfunction, increased activity could be appropriate and not due to lack of dopaminergic inhibition.
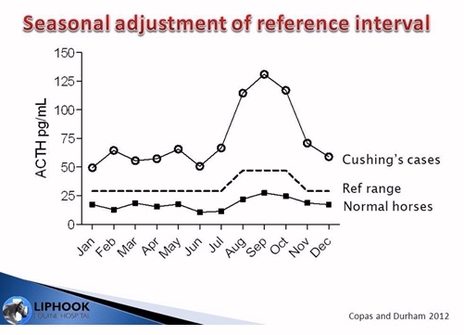
Autumn natural stimulation of ACTH
The pars intermedia becomes more active in the autumn in all horses, with increased hormone output and histological changes. It isn’t known exactly why this happens, but it may help the horse prepare for winter by changing metabolism and stimulating hair coat growth, as is seen in other species.
The difference between PPID and normal horse resting ACTH is greatest in the autumn, making this the best time to test.
Miller MA, Pardo ID, Jackson LP, Moore GE, Sojka JE
Correlation of Pituitary Histomorphometry with Adrenocorticotrophic Hormone Response to Domperidone Administration in the Diagnosis of Equine Pituitary Pars Intermedia Dysfunction
Vet Pathol 45:26–38 (2008)
Findings:
Sensitivity (true positives) Specificity (true negatives)
Alpha-MSH 63% 90%
ACTH 71% 81%
DST 65% 98%
All 3 tests identified advanced (grade 5) PPID.
All 3 tests were poor at identifying early PPID.
ACTH had a weaker correlation with pars intermedia enlargement than Alpha-MSH or DST.
Alpha-MSH had 2/48 false positives, ACTH had 13/48 false positives.
ACTH may be from the pars intermedia or the pars distalis, and may increase with other diseases or stress.
Resting cortisol is not predictive of PPID (this has been known for a long time).
A better test was needed.
Since around 2011 the TRH stimulation of ACTH has been used to diagnose PPID, and further research is being carried out on this test.
The vet collects a blood sample to measure resting ACTH, then injects 1 mg of the hormone TRH intravenously and collects blood to measure ACTH 10 and/or 30 mins after giving the TRH.
Currently suggested reference ranges are:
PPID if ACTH > 36 pg/ml (using Immulite CIA) at 0 or 30 minutes
PPID if ACTH > 110 pg/ml at 10 minutes
However these reference ranges are likely to change as more data is collected (normal horses have tested above these ranges), and reference ranges have not been established for the autumn seasonal rise.
Sensitivity (true positives) 88-95%, specificity (true negatives) 71-91% so the TRH stimulation of ACTH appears more diagnostic than resting ACTH.
TRH stimulates pars intermedia activity – however, increased activity does not necessarily mean dysfunction, increased activity could be appropriate and not due to lack of dopaminergic inhibition.
Autumn natural stimulation of ACTH
The pars intermedia becomes more active in the autumn in all horses, with increased hormone output and histological changes. It isn’t known exactly why this happens, but it may help the horse prepare for winter by changing metabolism and stimulating hair coat growth, as is seen in other species.
The difference between PPID and normal horse resting ACTH is greatest in the autumn, making this the best time to test.
Copas VEN, Durham AE
Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction
Equine Veterinary Journal Vol 44, Issue 4, pages 440–443, July 2012
EMS
EMS is not a disease, it is a cluster of risk factors which indicate that a horse is at greater risk of developing endocrinopathic laminitis.
Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ
Equine Metabolic Syndrome - ACVIM Consensus Statement
J Vet Intern Med 2010;24:467–475
The 2010 ACVIM Consensus Statement described the EMS phenotype as including:
Plus horses may be described as “easy keepers” or “thrifty”, mares may have abnormal reproductive seasons, blood tests for lipids and leptin may show abnormalities. Some breeds appear to be more predisposed to EMS than others.
Insulin Dysfunction - Insulin Resistance v Hyperinsulinaemia
Insulin resistance is when the body produces insulin, but the insulin sensitive tissue (primarily muscle) does not respond normally to that insulin, which prevents glucose in the blood from entering the tissue normally.
Insulin resistance is at the level of the tissue (muscle).
Over time, insulin resistance causes the horse to “compensate” by making more insulin to ensure that glucose does enter the tissue – this is compensatory hyperinsulinaemia .
However, chronic hyperglycaemia and hyperinsulinaemia may be the primary problem, and can lead to tissue insulin resistance.
Why is this important?
It is hyperinsulinaemia (high concentrations of insulin), not insulin resistance, that is the risk factor for endocrinopathic laminitis, and this has implications for both testing and treatment.
For example, if the primary problem is at the level of the pancreas, not the level of the tissue, and a drug is given that causes improvement at the tissue level, the health of that animal may not be improved.
Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction
Equine Veterinary Journal Vol 44, Issue 4, pages 440–443, July 2012
EMS
EMS is not a disease, it is a cluster of risk factors which indicate that a horse is at greater risk of developing endocrinopathic laminitis.
Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ
Equine Metabolic Syndrome - ACVIM Consensus Statement
J Vet Intern Med 2010;24:467–475
The 2010 ACVIM Consensus Statement described the EMS phenotype as including:
- General (obesity = body condition score (BCS) of 7 to 9 on the 9 point scale) or regional adiposity (crest of neck, tailhead, shoulder, sheath/mammory glands),
- Hyperinsulinaemia/abnormal insulin response,
- Predisposition to laminitis.
Plus horses may be described as “easy keepers” or “thrifty”, mares may have abnormal reproductive seasons, blood tests for lipids and leptin may show abnormalities. Some breeds appear to be more predisposed to EMS than others.
Insulin Dysfunction - Insulin Resistance v Hyperinsulinaemia
Insulin resistance is when the body produces insulin, but the insulin sensitive tissue (primarily muscle) does not respond normally to that insulin, which prevents glucose in the blood from entering the tissue normally.
Insulin resistance is at the level of the tissue (muscle).
Over time, insulin resistance causes the horse to “compensate” by making more insulin to ensure that glucose does enter the tissue – this is compensatory hyperinsulinaemia .
However, chronic hyperglycaemia and hyperinsulinaemia may be the primary problem, and can lead to tissue insulin resistance.
Why is this important?
It is hyperinsulinaemia (high concentrations of insulin), not insulin resistance, that is the risk factor for endocrinopathic laminitis, and this has implications for both testing and treatment.
For example, if the primary problem is at the level of the pancreas, not the level of the tissue, and a drug is given that causes improvement at the tissue level, the health of that animal may not be improved.
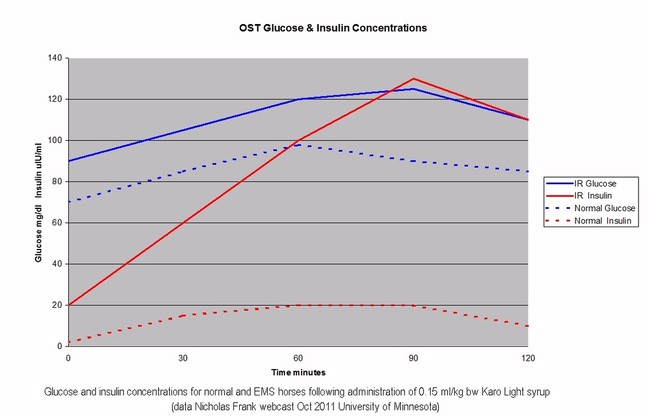
When a normal horse eats, glucose is absorbed into the blood stream and insulin is released to enable that glucose to enter insulin sensitive tissue – blood levels of both glucose and insulin rise after a meal and then drop back down (dotted lines above).
When an EMS horse eats, glucose is absorbed into the blood stream and insulin is released, but if the horse has a problem at the level of the insulin sensitive tissue, the glucose doesn’t enter the tissue efficiently, so the pancreas compensates by making more insulin – blood levels of both glucose and insulin increase and take longer to drop back down (solid lines above).
If a horse has greater than normal absorption of glucose or greater than normal release of insulin from the pancreas, over time the horse can develop insulin resistance at the level of the tissue - a dynamic oral test will pick up dysfunction at every level.
Diagnosis of insulin dysregulation
The fasting blood insulin concentration is the easiest test to carry out and involves taking a single sample of blood after the horse has been fasted for at least 6 hours.
An above normal result (20 µIU/ml is often used as the cut-off but see below) is diagnostic of hyperinsulinaemia. The test has a high false negative rate (around 2/3 of horses with insulin dysregulation have normal fasting insulin), so a normal test result does not rule out EMS, and a dynamic test should follow a normal fasting insulin test for any horse suspected of having insulin dysregulation.
Glucose may be normal or above normal.
Reference ranges for insulin may be breed specific, therefore a cut-off of 20 µIU/ml may not be appropriate for all breeds.
Reference ranges for insulin are laboratory specific, therefore a cut-off of 20 µIU/ml may not be appropriate for all labs (different assays, e.g. RIA v CIA, may produce different results meaning that results cannot be compared).
Dr McFarlane suggests that PPID horses with insulin resistance more commonly have abnormal fasting insulin results than horses with EMS only.
The most appropriate dynamic test is the oral sugar test (OST) – this mimics natural conditions, testing at the level of the GI tract, pancreas and tissue, not just at the level of the tissue.
The horse is fasted for at least 6 hours, then given 0.15 ml/kg bodyweight (so 75 ml for a 500 kg horse) Karo Light syrup by mouth (either syringed directly in to the mouth or in a small low sugar feed). Blood is collected 60 and 90 minutes later and both insulin and glucose are measured.
Insulin > 60 µIU/ml at 60 or 90 minutes is diagnostic of hyperinsulinaemia.
Insulin between 45-60 µIU/ml at 60 or 90 minutes is equivocal and further testing should be considered.
Insulin below 45 µIU/ml at 60 or 90 minutes is considered normal.
Glucose > 125 mg/dl at 60 or 90 minutes is considered an excessive glucose response.
(For more details see the Equine Endocrinology Group Recommendations for the diagnosis and treatment of PPID).
The combined glucose insulin tolerance test (CGITT) and insulin tolerance test (ITT) measure insulin resistance at the level of the tissue only (NB the ITT may risk causing hypoglycaemia).
However, EMS is a very frustrating condition and the current tests are not that good. Vets are seeing horses they strongly suspect have insulin dysregulation but are having trouble getting the test results to prove it.
Dr McFarlane looked at horses that appeared to be hyperinsulinaemic, all had a BCS of 8 or 9 and two thirds of them had foundered, but she could not get half of these horses to test positive.
She suggests if there is a clinical indication of a “thrifty” horse then it’s best to consider that it has EMS and treat appropriately.
The purpose of trying to diagnose EMS is to prevent laminitis.
Endocrinopathic laminitis
Endocrine disease (obesity/EMS, PPID) is the most common cause of laminitis, and may be triggered by diet, particularly when grasses are highest in NSC.
Endocrinopathic laminitis can be insidious and may be mistaken for other conditions - radiographic and hoof growth changes may precede clinical symptoms.
Endocrinopathic laminitis was once believed to be the result of excessive cortisol, but there is now strong evidence that it is caused by serum insulin concentration (hyperinsulinaemia), as horses receiving a continuous infusion of insulin consistently develop laminitis.
Endocrine laminitis has different histological characteristics to SIRS laminitis (laminitis secondary to endotoxaemia e.g. colitis, retained placenta). With endocrine laminitis, the secondary epidermal laminae (SEL) become longer and narrower before clinical signs of laminitis are seen, and there is a general absence of inflammation (compared to SIRS laminitis). It is currently thought that excessive insulin binds to and activates insulin growth factor–1 (IGF-1) receptors on the laminae causing cell division and elongation of the SEL, making them structurally unsound and causing laminitis.
Potential new drugs for the treatment and/or prevention of endocrinopathic laminitis may target this area.
EMS and PPID Summary
EMS and PPID are not mutually exclusive – a horse can have EMS and PPID.
Clinical signs common to EMS and PPID include laminitis, hyperinsulinaemia, abnormal fat deposits, PU/PD, abnormal sweating, exercise intolerance, infertility.
EMS by definition is hyperinsulinaemia or an excessive insulin response to a meal stimulus (which increases the risk of laminitis).
PPID by definition is an overly active pars intermedia.
Horses with EMS may be at greater risk of developing PPID as they get older – horses with EMS should be monitored and tested for PPID.
There appears to be a transitional period between the diseases during which horses have both EMS and PPID at the same time. These horses often have higher insulin concentrations and potentially a greater risk of laminitis, therefore treatment should be instigated as early as possible.
It isn’t yet known whether EMS and PPID are causitively linked – i.e. whether having EMS causes PPID.
In Dr McFarlane’s experience, a horse with PPID that has never had laminitis and has normal insulin is not at greater risk of developing laminitis than any other horse.
Equine endocrine diseases are progressive and difficult to diagnose in the early stages – there will always be a grey zone for testing in the early stages of a progressive disease. Retest horses that are suspected of having EMS/PPID if they have negative results, and/or instigate treatment.
History and clinical signs are essential for early diagnosis – a diagnosis should not be made on the basis of diagnostic test results if there are no clinical signs.
Tests can be carried out in any season as long as results are interpreted correctly, and autumn is the best time to test for PPID using resting ACTH.
Insulin/insulin dynamics should be measured in all equine endocrine cases (PPID as well as EMS) as insulin dysfunction is predictive of laminitis risk.
Use resting ACTH concentration or TRH stimulation of ACTH for PPID diagnosis.
Use fasting insulin or the oral sugar test to assess risk of laminitis (for both EMS and PPID).
Believe clinical impressions, retest or treat cases that are suspicious – people are better at diagnosing PPID (and EMS) than current blood tests are at confirming it. However, always get blood tests done too to monitor disease progression/efficacy of treatment.
The goal is to recognise these diseases early to avoid clinical signs, especially laminitis – early intervention may keep horses healthy for longer.
When an EMS horse eats, glucose is absorbed into the blood stream and insulin is released, but if the horse has a problem at the level of the insulin sensitive tissue, the glucose doesn’t enter the tissue efficiently, so the pancreas compensates by making more insulin – blood levels of both glucose and insulin increase and take longer to drop back down (solid lines above).
If a horse has greater than normal absorption of glucose or greater than normal release of insulin from the pancreas, over time the horse can develop insulin resistance at the level of the tissue - a dynamic oral test will pick up dysfunction at every level.
Diagnosis of insulin dysregulation
The fasting blood insulin concentration is the easiest test to carry out and involves taking a single sample of blood after the horse has been fasted for at least 6 hours.
An above normal result (20 µIU/ml is often used as the cut-off but see below) is diagnostic of hyperinsulinaemia. The test has a high false negative rate (around 2/3 of horses with insulin dysregulation have normal fasting insulin), so a normal test result does not rule out EMS, and a dynamic test should follow a normal fasting insulin test for any horse suspected of having insulin dysregulation.
Glucose may be normal or above normal.
Reference ranges for insulin may be breed specific, therefore a cut-off of 20 µIU/ml may not be appropriate for all breeds.
Reference ranges for insulin are laboratory specific, therefore a cut-off of 20 µIU/ml may not be appropriate for all labs (different assays, e.g. RIA v CIA, may produce different results meaning that results cannot be compared).
Dr McFarlane suggests that PPID horses with insulin resistance more commonly have abnormal fasting insulin results than horses with EMS only.
The most appropriate dynamic test is the oral sugar test (OST) – this mimics natural conditions, testing at the level of the GI tract, pancreas and tissue, not just at the level of the tissue.
The horse is fasted for at least 6 hours, then given 0.15 ml/kg bodyweight (so 75 ml for a 500 kg horse) Karo Light syrup by mouth (either syringed directly in to the mouth or in a small low sugar feed). Blood is collected 60 and 90 minutes later and both insulin and glucose are measured.
Insulin > 60 µIU/ml at 60 or 90 minutes is diagnostic of hyperinsulinaemia.
Insulin between 45-60 µIU/ml at 60 or 90 minutes is equivocal and further testing should be considered.
Insulin below 45 µIU/ml at 60 or 90 minutes is considered normal.
Glucose > 125 mg/dl at 60 or 90 minutes is considered an excessive glucose response.
(For more details see the Equine Endocrinology Group Recommendations for the diagnosis and treatment of PPID).
The combined glucose insulin tolerance test (CGITT) and insulin tolerance test (ITT) measure insulin resistance at the level of the tissue only (NB the ITT may risk causing hypoglycaemia).
However, EMS is a very frustrating condition and the current tests are not that good. Vets are seeing horses they strongly suspect have insulin dysregulation but are having trouble getting the test results to prove it.
Dr McFarlane looked at horses that appeared to be hyperinsulinaemic, all had a BCS of 8 or 9 and two thirds of them had foundered, but she could not get half of these horses to test positive.
She suggests if there is a clinical indication of a “thrifty” horse then it’s best to consider that it has EMS and treat appropriately.
The purpose of trying to diagnose EMS is to prevent laminitis.
Endocrinopathic laminitis
Endocrine disease (obesity/EMS, PPID) is the most common cause of laminitis, and may be triggered by diet, particularly when grasses are highest in NSC.
Endocrinopathic laminitis can be insidious and may be mistaken for other conditions - radiographic and hoof growth changes may precede clinical symptoms.
Endocrinopathic laminitis was once believed to be the result of excessive cortisol, but there is now strong evidence that it is caused by serum insulin concentration (hyperinsulinaemia), as horses receiving a continuous infusion of insulin consistently develop laminitis.
Endocrine laminitis has different histological characteristics to SIRS laminitis (laminitis secondary to endotoxaemia e.g. colitis, retained placenta). With endocrine laminitis, the secondary epidermal laminae (SEL) become longer and narrower before clinical signs of laminitis are seen, and there is a general absence of inflammation (compared to SIRS laminitis). It is currently thought that excessive insulin binds to and activates insulin growth factor–1 (IGF-1) receptors on the laminae causing cell division and elongation of the SEL, making them structurally unsound and causing laminitis.
Potential new drugs for the treatment and/or prevention of endocrinopathic laminitis may target this area.
EMS and PPID Summary
EMS and PPID are not mutually exclusive – a horse can have EMS and PPID.
Clinical signs common to EMS and PPID include laminitis, hyperinsulinaemia, abnormal fat deposits, PU/PD, abnormal sweating, exercise intolerance, infertility.
EMS by definition is hyperinsulinaemia or an excessive insulin response to a meal stimulus (which increases the risk of laminitis).
PPID by definition is an overly active pars intermedia.
Horses with EMS may be at greater risk of developing PPID as they get older – horses with EMS should be monitored and tested for PPID.
There appears to be a transitional period between the diseases during which horses have both EMS and PPID at the same time. These horses often have higher insulin concentrations and potentially a greater risk of laminitis, therefore treatment should be instigated as early as possible.
It isn’t yet known whether EMS and PPID are causitively linked – i.e. whether having EMS causes PPID.
In Dr McFarlane’s experience, a horse with PPID that has never had laminitis and has normal insulin is not at greater risk of developing laminitis than any other horse.
Equine endocrine diseases are progressive and difficult to diagnose in the early stages – there will always be a grey zone for testing in the early stages of a progressive disease. Retest horses that are suspected of having EMS/PPID if they have negative results, and/or instigate treatment.
History and clinical signs are essential for early diagnosis – a diagnosis should not be made on the basis of diagnostic test results if there are no clinical signs.
Tests can be carried out in any season as long as results are interpreted correctly, and autumn is the best time to test for PPID using resting ACTH.
Insulin/insulin dynamics should be measured in all equine endocrine cases (PPID as well as EMS) as insulin dysfunction is predictive of laminitis risk.
Use resting ACTH concentration or TRH stimulation of ACTH for PPID diagnosis.
Use fasting insulin or the oral sugar test to assess risk of laminitis (for both EMS and PPID).
Believe clinical impressions, retest or treat cases that are suspicious – people are better at diagnosing PPID (and EMS) than current blood tests are at confirming it. However, always get blood tests done too to monitor disease progression/efficacy of treatment.
The goal is to recognise these diseases early to avoid clinical signs, especially laminitis – early intervention may keep horses healthy for longer.

 RSS Feed
RSS Feed