Meyer JC, Hunyadi LM, Ordóñez-Mena JM
The accuracy of ACTH as a biomarker for Pituitary Pars Intermedia Dysfunction in horses: A systematic review and meta-analysis
Equine Vet J. 2021 Aug 24. doi: 10.1111/evj.13500. Epub ahead of print. PMID: 34428330
Conclusions: !"In horses with a high pre-test probability of PPID, ACTH may be a functional "rule-in" test. Baseline ACTH is not recommended for screening purposes or use in horses without clinical signs of PPID."
See also A Systematic Review and Meta-analysis on the diagnosic accuracy of baseline ACTH for the diagnosis of PPID in adult horses and ponies by James Meyer 4th Global Equine Endocrine Symposium 2020 p8
Conclusions: "The overall results and those in the reference standard subgroup of histopathology suggest that the specificity of baseline ACTH for the diagnosis of PPID is good while the sensitivity is marginal (lower CI 57%). This would corroborate the current recommendation that baseline ACTH be used as a triage test for PPID with further diagnostics being recommended in patients that test negative."

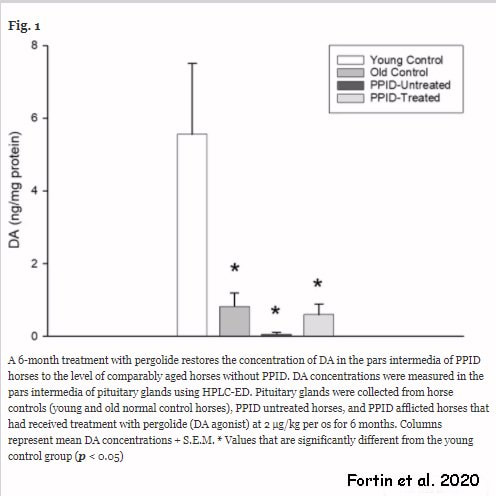
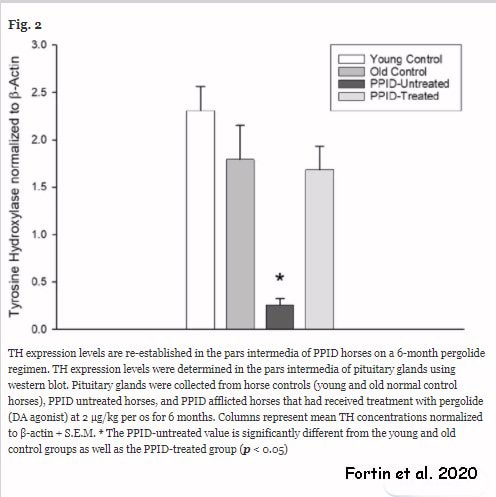

 RSS Feed
RSS Feed